
Does this news mean the Pfizer vaccine is safe for all?
In what seems to be a breakthrough in exciting COVID vaccine news, Pfizer has officially announced that its vaccine, first given emergency authorization at the tail end of 2020, is both safe & effective for kids between the ages of five and eleven. The pharmaceutical companies said the early results of their trial indicate the vaccine is safe for children and establishes a strong antibody response against the virus. Amazing!
Giving a two-dose regimen administered twenty-one days apart for children between five and eleven years old was well taken, according to Pfizer & BioNTech. Side effects were also generally comparable to those of people between the ages of sixteen and twenty-five years old who received the shot. This is a good sign considering that the U.S. is trying to get to the high percentage vaccination number to establish herd immunity.
This is exciting Pfizer news, no doubt about it. However, with the ever-surging delta variant and a slew of COVID breakthrough cases, is this too little too late? Let’s dive into the full story from Pfizer & BioNTech and see what the latest update is.

Pfizer gives the go-ahead for children
“Since July, pediatric cases of COVID-19 have risen by about 240 percent in the U.S., underscoring the public health need for vaccination. These trial results provide a strong foundation for seeking authorization of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency,” said Albert Bourla, the chairman and CEO for Pfizer.
“Over the past nine months, hundreds of millions of people ages 12 and older from around the world have received our COVID-19 vaccine,” Bourla continued. “We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorization, especially as we track the spread of the Delta variant and the substantial threat it poses to children.”
While this is certainly promising news for parents who wish to get their children aged 5+ vaccinated, it still may be some time before the vaccine gets the official rollout, as Pfizer will have to submit the results of the trial to the FDA to review for emergency use. The current estimation is somewhere around late 2021. As well, trial results for children under five could also come later this year.

“Already in March 2021, we have started the study to evaluate the immunization of younger children. Our objective was to generate and submit the data for schoolkids to regulatory authorities around the world before the winter season begins,” BioNTech CEO and co-founder Ugur Sahin said.
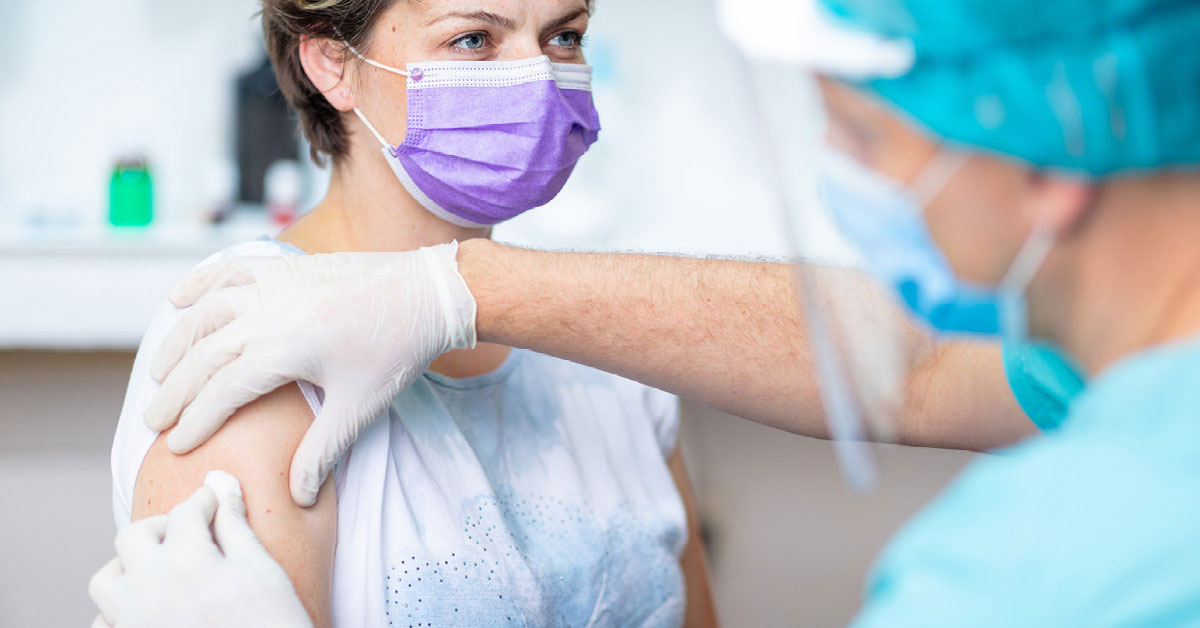
U.S. vaccines available
According to recent data released by the Centers for Disease Control and Prevention, over 51% of U.S. citizens have been fully vaccinated for the coronavirus. As of today, there are three vaccines that are being used to fight the spread of COVID-19 and end the current global pandemic.
The first vaccine, developed by Pfizer-BioNTech, was first administered in the U.S. at the end of 2020. The second vaccine to fight COVID-19 was developed by Moderna, which was also first given the green light for emergency use at the tail-end of 2020. Both of these coronavirus vaccines are estimated to combat the coronavirus with an impressive effectiveness rate of over 93%.
However, both vaccines currently require two separate doses, with each needing several weeks in between to build the proper antibodies for protection against the deadly disease. A third coronavirus vaccine, this time developed by Johnson & Johnson, was given the OK in early 2021. This vaccine, while not as efficient as Pfizer & Moderna, is said to have an effectiveness rate of over 85%, and requires only a single shot.






