Test for coronavirus at home: Does the FDA-approved kit really work?
The COVID-19 pandemic has claimed over 1.34 million lives so far. That’s an astonishing number by any standard. The US reported more than 187,000 new cases of infection from the coronavirus on November 19. This is the highest one-day figure since the pandemic began. At the same time, in India, the other country paying a huge price for the pandemic surpassed 9 million cases.
Some models have tweaked their forecasts to reflect how the situation is getting progressively worse, predicting 471,000 deaths by March next year. As the pandemic has evolved, it’s become increasingly harder to access healthcare – which was seriously bad news for testing of the infection –making it a priority for scientists & medical experts to create a testing kit that can be used at home.

Testing for the infection
According to guidelines issued by Centers for Disease Control & Prevention (CDC), people who have symptoms of COVID-19 or people who’ve had close contact with someone with confirmed COVID-19 should get tested for the infection. Symptoms of infection include fever, dry cough, and dryness. In the most serious cases, people have been reporting difficulty in breathing, chest pain, or even mobility issues.
Most commonly, infections are also being caught if any combination of aches and pains, sore throat, diarrhea, conjunctivitis, headache, loss of taste or smell, a rash on the skin, or discoloration of fingers or toes exist together.
Close contact involves interaction or touch within 6 feet for a total of 15 minutes or more. The group of people often in contact with possible infected individuals include people who were hospitalized or are about to be hospitalized for a procedure, healthcare workers & other frontline workers like first responders, people in group living situations, among others.

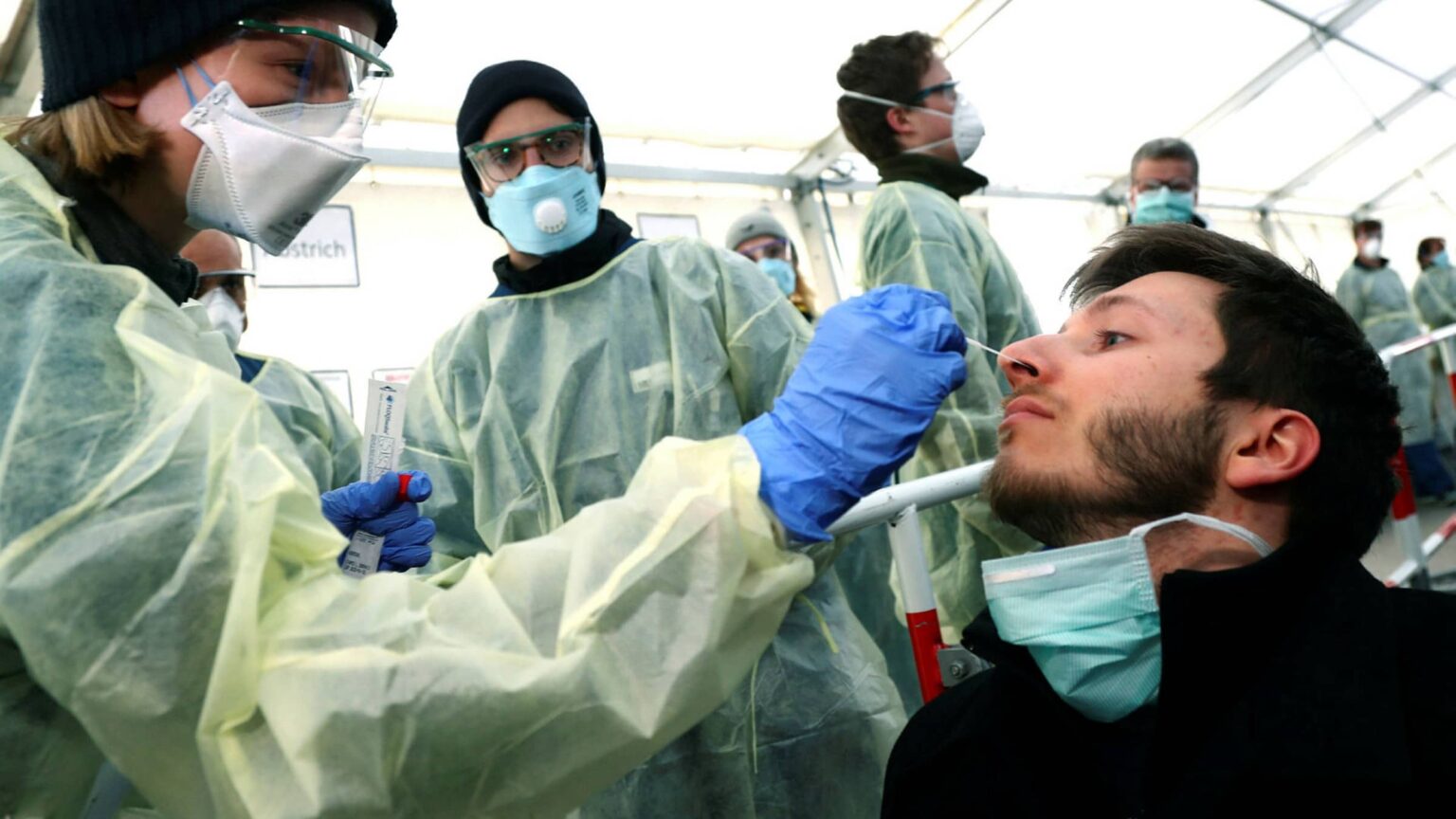
Available tests for the coronavirus
Two kinds of tests available for COVID-19 include the viral tests – done to determine if the infection exists, and antibody tests – done to determine if the individual has already had an infection in the past.
The viral test involves an uncomfortable test – insertion of a 60inch long swab – akin to a Q-tip – into the cavity between your nose & mouth. This swab is to be rotated there for 15 seconds. The same process is repeated on both sides to ensure proper sample collection. So far, this swab is being sent to a lab for testing after being inserted into a container.
The tests arrive from the lab & you know the drill. If you test positive, self-isolation & other preventative & safety steps are to be taken. A negative test shouldn’t make you callous either, so the precautions are to continue.

New testing options on the horizon
With the magnitude of cases, having a testing kit that can be used at home will help. Earlier this week, the Food and Drug Administration (FDA) granted emergency use authorization for the 30-minute kit manufactured by Lucira Health. The test kit can only be used after a prescription. Additionally, it’s been approved for only possible patients aged 14 & older.
In a statement issued by the FDA, Commissioner Dr. Stephen Hahn shares, “This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission. Today’s action underscores the FDA’s ongoing commitment to expand access to COVID-19 testing.”
This test is easy to follow, too. The nasal swab is to be placed into a vial plugged into a portable testing unit. The device has been trained to display if the results are positive or negative for infection in a time span of 30 minutes.
—
The impact of at-home testing kits, according to experts, will be seen in the form of curbing the spread of the infection in medical centers & testing hotspots, as well as help to massively reduce the turnaround time to get the test reports.



